-
Waylon J. Hastings
- Assistant Professor
- Office:
- 145 Norman E. Borlaug Building, College Station, Texas
- Email:
- [email protected]
Education
- Undergraduate Education
- B.S. Biochemistry & Genetics, Texas A&M University (2013)
- B.A. Mathematics, Texas A&M University (2013)
- Graduate Education
- M.S. Educational Administration, Texas A&M University (2015)
- Ph.D. Biobehavioral Health & Bioethics, Pennsylvania State University (2020)
- Awards
- Neahous-Shepardson Faculty Development Scholarship, Texas A&M University (2024)
- Butler-Williams Scholar, National Institute on Aging (2021)
- T32 Graduate Fellow, Penn State University Center for Healthy Aging (2018-2020)
- Penn State University Distinguished Graduate Fellow (2015-2017)
- Texas A&M Student Affairs Graduate Assistant of the Year (2015)
- Courses Taught
- NUTR 681 Graduate Seminar in Nutrition
- NUTR 481 Critical Appraisal of Nutrition Literature
- NUTR 412 Nutritional Treatment of Disease
Professional Summary
I collaborate with intramural/extramural entities including the Telomere Research Network (TRN), CALERIE™ Clinical Trial, and COnsortium of METabolomics Studies (COMETS), as well as industry partners (Bayer AG, Hurdle.Bio/Chromomics Ltd, Bryleos) to develop efficient, scalable approaches to track individual differences in aging and disease. In doing so, I aim to improve the effectiveness of interventions aimed at increasing healthy lifespans, progress toward which has been highlighted by Science Daily, the American Council for Science and Health, and the New York Post.
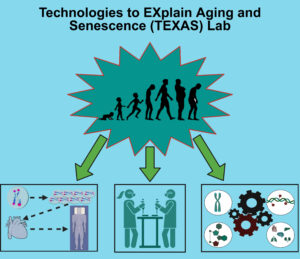
Our work in the Technologies to EXplain Aging and Senescence (TEXAS) Lab takes a three-pronged approach to optimize multi-dimensional measures of human aging and functional decline, with an emphasis on telomere biology. From a biostatistical standpoint, we leverage existing data from large-scale cohort studies to validate metrics of biological aging. This includes examining their ability to predict cognitive and physical function (1-2), testing their responsiveness to life course risk exposures such as food insecurity, poverty, and ‘costs of reproduction’ (3), and examining their utility as surrogate endpoints within gero-protective interventions like caloric restriction and nutraceutical supplementation (4-6). Our current projects include collaborations with COMETS to generate a novel measurement of metabolomic aging (7) and work with Hurdle to explore novel biomarkers of “inflammaging” with direct-to-market translation (8).
Bench science remains central to our research. As a graduate student, (9-11) and Telomere Research Network-supported post-doc (12-13), I spent over a decade of research investigating factors contributing to methodological variation in telomere measurements. Leveraging this expertise, we implement rigorously validated protocols to perform an array of quantification assays (e.g., immunoassay, qPCR, RNAseq) on a variety of biospecimens (e.g., culture, blood, urine, saliva), and are currently generating a novel assay for chromosome-specific telomere characterization.
Inspired by an innovative study design produced by my PhD advisor Dr. Idan Shalev (14), the final dimension of work in the TEXAS Lab involves translating the rigor of animal model research into human clinical studies. Toward this end, we are implementing clinical studies to probe the robustness of aging measurements to transient metabolic, neuroendocrine, and immunogenic stressors (15).
Selected Publications
- Hastings WJ, Shalev I, & Belsky DW (2019). Comparability of biological aging measures in the National Health and Nutrition Examination Study, 1999-2001. Psychoneuroendocrinology, 106, 171-178, doi: 10.1016/j.psyneuen.2019.03.012.
- Hastings WJ, Almeida DM, & Shalev I (2021) Conceptual and analytical overlap between allostatic load and systemic biological aging measures: Analyses from the National Survey of Midlife Development in the United States. Journals of Gerontology Series A. doi: 10.1093/Gerona/glab187.
- Shirazi TN*, Hastings WJ*, Rosinger AY, & Ryan CP (2020) Parity predicts biological age acceleration in post-menopausal women: Evidence from NHANES 1999-2010. Scientific Reports, 10(1), 1-13. doi: 10.1038/s41598-020-77082-2.
- Waziry R, Ryan CP, Corcoran DL, Huffman KM, Kobor MS, Kothari M, Graf GH, Kraus VB, Kraus WE, Lin DTS, Pieper CF, Ramaker ME, Bhapkar M, Das SK, Ferrucci L, Hastings WJ, Kebbe M, Parker DC, Racette SB, Shalev I, Schilling B, & Belsky DW (2023) Effect of long-term caloric restriction on DNA methylation measures of biological aging in healthy adults: CALERIE Trail Analysis. Nature Aging. doi: 10.1038/s43587-022-00357-y.
- Hastings WJ, Ye Q, Wolf S, Ryan C, Das SK, Huffman KM, Kobor MS, Kraus WE, MacIsaac JL, Martin CK, Racette SB, Redman LM, Belsky DW, & Shalev I (2024). Effect of long-term caloric restriction on telomere length in healthy adults: CALERIE™ trial analysis. Aging Cell. doi: 10.1111/acel.14149.
- Hastings WJ, McGrath L, & Komac W (Oct. 2025) Translational pharmacodynamics of lathmized NAD+ in aging and neurodegenerative disease. Biomarkers of Aging Consortium. Boston, MA.
- Hastings WJ (May 2024) Leveraging COMETS to explore impacts of caloric restriction on metabolomic aging. Consortium of Metabolomic Studies NCI/NCATS Virtual Workshop. Online.
- Schmunk LJ, Call TP, McCartney DL, Javaid H, Hastings WJ, Jovicevic W, Kojadinovic D, Tomkinson N, Zlamalova E, McGee K, Sullivan J, Ovari V, Wishart K, Behrens C, Stone E, Gavrilov M, Thompson R, Jackson T, Lord JM, Stubbs TM, Marioni RE, Martin-Herranz DE (2025) A novel framework to build biologically interpretable saliva-based DNA methylation biomarkers: quantifying systemic chronic inflammation as a case study. Aging Cell. doi: 10.111/acel.14444.
- Hastings WJ, Shalev I, & Belsky DW (2017). Translating measures of biological aging to test effectiveness of geroprotective interventions: What can we learn from research on telomeres?. Frontiers in Genetics, 8, 164, doi: 10.3389/fgene.2017.00164.
- Hastings WJ, Eisenberg DTA, & Shalev I (2021) Impact of amplification efficiency approaches on telomere length measurement via qPCR. Frontiers in Genetics. doi:10.3389/fgene.2021.728603.
- Hastings WJ, Eisenberg DTA, & Shalev I (2020) Uninterruptible power supply improves precision and external validity of telomere length measurement via qPCR. Experimental Results. doi: 10.1017/exp.2020.58.
- Wolf SE, Hastings WJ, Ye Q, Etzel L, Apsley AT, Chiaro C, Heim CM, Heller T, Noll JG, O’Donnell KJ, Schreier HMC, Shenk CE, Shalev I, (2024) Cross-tissue comparison of telomere length and DNA quality metrics among individuals aged 8 to 70 years. PLOS One. doi:10.1371/journal.pone.0290918.
- Lin J, Verhulst S, Fernandez Alonso C, Dagnall C, Shahinaz G, Hastings WJ, Lai TP, Shalev I, Wang Y, Zheng, YL, Epel E, & Drury SS (2022). Effects of DNA extraction, DNA integrity, and laboratory on the precision of qPCR-based telomere length measurement – a multi-lab impartial study. bioRxiv. doi: 10.1101/2022.12.14.520438.
- Shalev I, Hastings WJ, Etzel L, Hendrick KA, Israel S, Russell M, Siegel SR, & Zinoble M (2020) Investigating the impact of early-life adversity on physiological, immune, and gene expression responses to acute stress: A pilot feasibility study. PLoS One 15(4), doi: 10.1371/journal.pone.0221310.
- Apsley AT, Ye Q, Etzel L, Wolf S, Hastings WJ, Mattern BC, Siegel SR, & Shalev I (2023) Biological stability of DNA methylation measurements over varying intervals of time and in the presence of acute stress. Epigenetics. doi: 10.1080/15592294.2023.2230686.